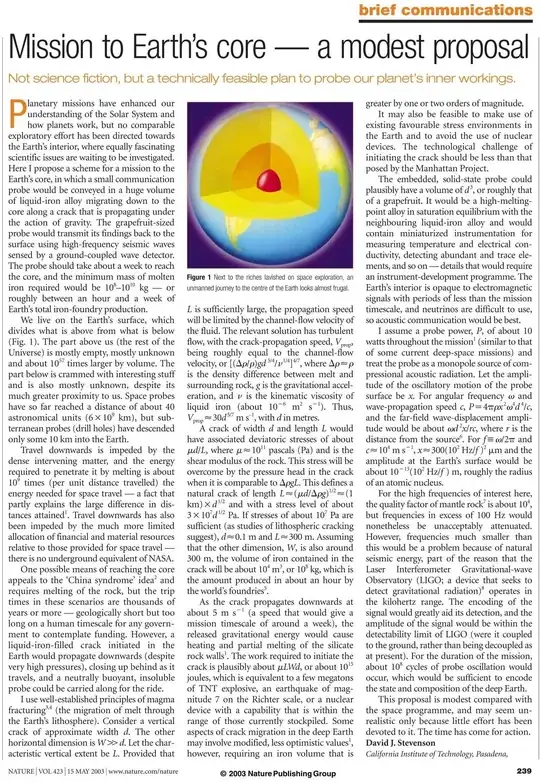
Nature communications article "Mission to Earth's core — a modest proposal", suggests placing a large volume of liquid iron in a crack and let it sink all the way to the Earth's core, carrying along a probe that can transmit data using seismic waves.
It is an interesting idea. Although there might be many technical problems I feel no one seems insurmountable and it is worth a try.
However, I wonder why the author suggest using a liquid iron instead of a liquid lead. I would expect lead to be cheaper and also require a smaller volume to achieve the same fracture stresses (due to higher density). In addition, a lower melting point would facilitate the initiation of the fracture propagation near the surface.
Would lead dissolve in the surrounding magma? Would it chemically interact turning into a lighter compound?
As the original article is behind a paywall I copy here the main text: