I know that on the moon, while the daytime surface temperature can be above 270° F, in the shade but a few feet above the surface temperature can get quite low. Wondering whether a similar phenomenon can be observed if a vaccum is created around an object which is suspended in he middle of it.
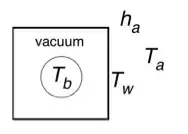
Say for example, a steel ball is suspended in the middle of a plexiglass cube, by 3 plexiglass threads, and the interior volume of the cube is 1 cubic meter, and the ball has a diameter of 10cm, the box walls are 5cm thick. The cube has been evacuated to the practical limit of modern vacuum pumps. Let's say the steel ball is chilled to a temperature of -20 c (measured at the time the evacuation is complete). Let's say it's 70° F outside, and the box is sitting on a plexiglass tripod in the shade, shielded from direct sunlight.
What would be the temperature reading of the steel ball, approximately, after say 2 weeks? (i.e. at equilibrium).
(I don't mean to be overly prescriptive, but trying to be sufficiently specific to avoid answers like "it depends on how thick the walls are" and similar)