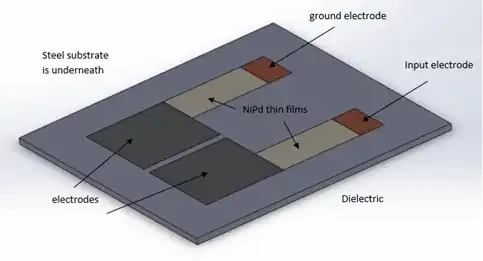
I’m designing a hydrogen and corrosion sensor. A thin film changes resistance based on hydrogen concentration. Then the capacitance between two electrodes will change depending on substrate thickness (corrosion). AC analysis can be used to see a phase change in the impedance, giving capacitance changes. I have designed a basic sensor, here it is with a basic model.
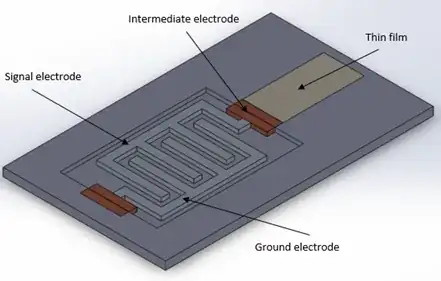
But now I think an interdigitated electrodes might be better to detect the change in corrosion from the steel substrate. I’ve thrown together a basic design. I’m not sure though, has anyone got any thoughts? And what would be the best material for this electrode, I was thinking a copper? Also what would be the best way to go about modelling interdigitated electrodes, assuming lots of parallel capacitors?
Many thanks