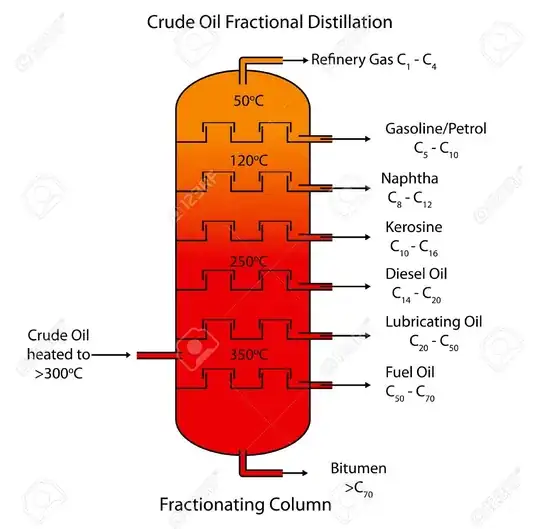
Does the plant starts by boiling crude oil in low temperature to extract the "lightest" product first,
and then subsequently increase it's temperature until achieve the "heaviest"?
Or all done in single batch, where it condensed separately?
From what I summarize from multiple video, it seems the latter - It's done in only one batch.
But how come? Since they are differ in boiling point. This really puzzled me