I made an electric furnace as a university project. It can heat up air inside up to 1000 degrees Celsius. It's not fully sealed so some air can escape from small gaps. Not sure if this would cause a problem or not later on, but water always drips a few times from certain places during operation.
The furnace is encased in a steel plate. I noticed that the top case has a lot of dew (from vapor condensation I assume) once the furnace started to heat up.
- What causes water to drip?
- Is it a dangerous thing?
- If it is, is there a way I can do to prevent it, or at least lessen the frequency?
IDK if this help, I live near the equator and the humidity here is always high.
Edit: added observation and pictures (read from top to bottom)
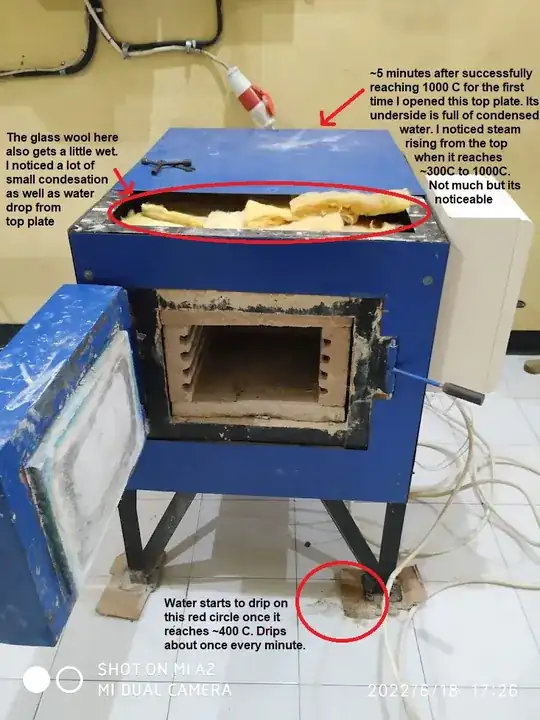
~5 minutes after successfully reaching 1000 C for the first time I opened this top plate. Its underside is full of condensed water. I noticed steam rising from the top when it reaches ~300C to 1000C. Not much but it's noticeable.